The topic of pH correction often triggers panic among beginners, making it seem complicated and too “scientific.” However, it’s not as daunting as it appears. It’s a routine operation, and the sooner you get the hang of it, the easier it will be for you to create and work with cosmetics.
pH – What Is It, Really? If you don’t have a background in chemistry, biology, or medicine, you’re not obliged to know about pH. But since we’re passionate about crafting cosmetics, it’s a concept worth understanding. You probably have a rough idea that pH measures how acidic or alkaline a product is. Advertisements might depict acidic pH as bad for teeth (hinting at potential cavities) and alkaline pH as bad for the skin. But what exactly is pH, and how is it measured?
In simple terms, pH is an indicator that can ONLY be measured in aqueous (water-based) solutions. It shows the prevalence of two types of ions in water or an aqueous solution:
- Hydrogen ions (H+): When these positive ions dominate, the solution becomes acidic. The more hydrogen ions, the lower the pH, making the solution more acidic.
- Hydroxide ions (OH-): When these negatively charged ions prevail, the solution becomes alkaline. More hydroxide ions mean a higher pH, indicating a stronger alkaline solution.
The pH scale looks like this:
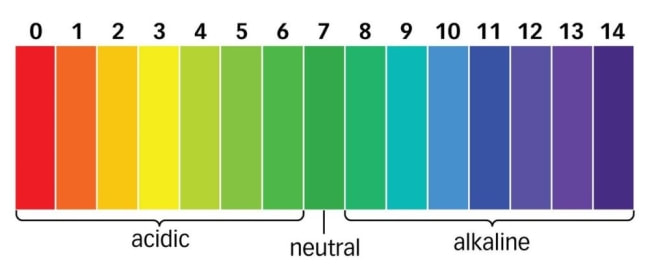
- pH 1 to 6: Acidic (dominance of H+ ions)
- pH 7: Neutral (balance of H+ and OH- ions)
- pH 7 to 14: Alkaline (dominance of OH- ions)
“But where do these ions come from in an aqueous solution?” you might ask.
Water is inherently neutral (or close to neutral). However, when we add acids to water, they release H+ ions, acidifying the solution and lowering its pH. The more acids in the water, the more H+ ions are released, making the solution more acidic.
Conversely, if we add alkalis or bases (like baking soda) to water, they dissolve and release OH- ions, alkalizing the solution and raising its pH.
When formulating cosmetics, we often aim to adjust the pH of our products to match the skin’s physiological pH, which is around 5 to 5.5. In some cases, the pH may need to be slightly lower (for example, hair conditioner requires a pH of 4.5-5) or higher (certain actives work best at pH 6-6.5, tear-free baby shampoo typically has a pH of 6.5-7, etc.).
Once the product-making process is complete, we measure its pH and, if necessary, make corrections using a BUFFER SOLUTION.

HOW TO ADJUST THE pH OF YOUR COSMETIC PREPARATIONS
So, we’ve reached the point where we need to discuss solutions or how to raise or lower the pH of your product. There’s nothing complicated here; you just need to decide what you’ll use as a pH buffer solution.
SOLUTIONS TO LOWER pH
In professional practice, concentrated solutions of lactic or citric acid are commonly used to lower the pH of a product. Lactic acid is available for purchase on different websites in solution form (typically 80-90% concentration), making it a ready-made pH adjuster. You can order it and use it directly.
Lactic acid has the added benefit of being a hydrating agent and can be used not only for pH correction but also as an active ingredient (for making lactic acid peels, for example).
Citric acid is available in the form of crystals at various places, including regular food stores. To use it as a pH adjuster, you’ll need to create a solution:
- 25 g distilled water
- 25 g citric acid
You can gently warm the solution in a water bath to help the citric acid dissolve better. Transfer the prepared solution into a bottle with a dropper and label it. You don’t need to preserve this solution as its low pH prevents microorganism growth.
SOLUTIONS TO RAISE pH
To raise pH, solutions of alkalis or amino acids are used, and you have a few options here. In DIY cream-making (especially in the early stages), you can create a 10% solution of baking soda, but it’s not the most convenient option because baking soda is a weak alkali, and you’d need to add a lot of its solution to adjust the pH. But you can start with baking soda:
- 9 g warm boiled water
- 1 g baking soda
Stir the solution until the baking soda fully dissolves. This solution is not stored; you introduce it into your product for correction and dispose of any leftovers. However, it’s better to eventually invest in slightly more concentrated solutions for easier use.
SODIUM HYDROXIDE SOLUTION
Sodium hydroxide is a strong alkali, and it’s often used for pH correction in cosmetic manufacturing. You can prepare a 10% sodium hydroxide solution:
- 1 g sodium hydroxide
- 9 g distilled water
Work with this solution while wearing protective masks and gloves! The resulting solution has a pH of around 12, and it doesn’t need preservation. Transfer it into a bottle with a dropper and label it.
L-ARGININE SOLUTION
L-arginine is an amino acid that can also be used for pH correction while providing skincare benefits. In my work, I use L-arginine for pH correction because it’s gentler than sodium hydroxide. It may be a bit harder to find, but it’s an excellent cosmetic ingredient!
L-arginine (make sure it’s a cosmetic-grade ingredient, not dietary supplements) is sold in powder form, and you’ll need to dissolve it in water:
- 1 g L-arginine
- 9 g distilled water
L-arginine doesn’t dissolve in water very easily, but patience and stirring will do the trick. Keep stirring until all the arginine flakes fully dissolve. The solution has a pH of 11-11.5, and it doesn’t require preservation. Transfer it into a bottle with a dropper and label it.
You’re done! Your buffer solutions can last a long time, but you’ll use them drop by drop, so there’s no need to make large quantities.
Remember that these solutions are the most caustic of all cosmetic ingredients in your DIY workshop. Therefore, ALWAYS STORE THEM OUT OF REACH OF CHILDREN, label them to avoid confusion with other components, and be sure to use protective gear when preparing them!
